Abstract
Introduction: Primary mediastinal large B-cell lymphoma (PMBCL) is a rare B-cell lymphoma that is typically reported in young females. Although previously considered as a subtype of diffuse large B-cell lymphoma, PMBCL has unique clinical and molecular characteristics. The optimal treatment option for PMBCL remains to be elucidated. Two regimens that have been used as frontline therapy are rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine and prednisone (R-CHOP) and rituximab, etoposide phosphate, prednisone, vincristine, cyclophosphamide and doxorubicin hydrochloride (R-EPOCH). Our study compares the efficacy and safety of R-CHOP and R-EPOCH in PMBCL.
Methods:
A systematic literature search was conducted on PubMed, Embase, Cochrane and Clinicaltrials.gov using MeSH terms and relevant keywords for PMBCL, R-CHOP and R-EPOCH, including trade names and generic names, from inception to July 2022. The inclusion criteria were clinical trials and observational studies comparing the efficacy and safety of R-CHOP with R-EPOCH in PMBCL. Efficacy was assessed in terms of overall response rate (ORR), complete response (CR), overall survival (OS), progression-free survival (PFS) and relapse. Safety was assessed in terms of risk of occurrence of neutropenia and febrile neutropenia. Estimates of risk ratio (RR) with 95% confidence intervals (95% CI) for dichotomous outcomes were pooled using the random effects model with the Mantel-Haenszel method. Forest plots were constructed to visualize the pooled results. Heterogeneity was estimated using the I2 statistic. All statistical analyses were performed in Review Manager software (RevMan, version 5.4.1).
Results:
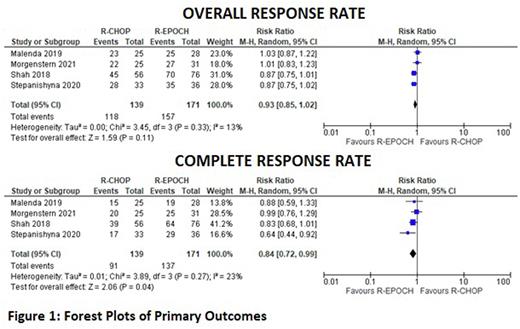
The initial search revealed 347 articles. After exclusion of duplicates, reviews and non-relevant articles, we included six articles (one prospective cohort and five retrospective cohorts) which compared outcomes following R-CHOP versus R-EPOCH in PMBCL. A total of 469 patients were included; R-CHOP and R-EPOCH were evaluated in 213 and 256 patients, respectively. The median age ranged from 27 to 35 years, and 318 (67.8%) patients were female. The median follow-up ranged from 23 to 60 months. R-CHOP and R-EPOCH were associated with an ORR of 84.9% and 91.8%, respectively. The difference between ORR of the two regimens was not statistically significant (RR 0.93, 95% CI 0.85-1.02; p = 0.11; I2 = 13%). However, R-CHOP was associated with significantly worse CR compared to R-EPOCH (65.5% versus 80.1%, RR 0.84, 95% CI 0.72-0.99; p = 0.04; I2 = 23%). OS was significantly lower with R-CHOP therapy (RR 0.84, 95% CI 0.72-0.97; p = 0.02; I2 = 36%). The two regimens had comparable PFS (RR 0.76, 95% CI 0.57-1.02; p = 0.06; I2 = 71%), and relapse rate (RR 0.91, 95% CI 0.26-3.16; p = 0.89; I2 = 56%). R-CHOP was associated with a lower risk of neutropenia (RR 0.67, 95% CI 0.54-0.83; p = 0.0004; I2 = 0%) and febrile neutropenia (RR 0.59, 95% CI 0.39-0.90; p = 0.01; I2 = 13%).
Conclusion:
R-EPOCH demonstrates superior CR and OS compared to R-CHOP. However, ORR, PFS and relapse rate were comparable for both regimens. R-CHOP was also associated with a lower risk of neutropenia and febrile neutropenia. Larger prospective studies and randomized controlled trials are required to establish the standard of care in PMBCL.
Disclosures
Anwer:Allogene Therapeutics: Research Funding; Janssen: Consultancy; BMS: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal